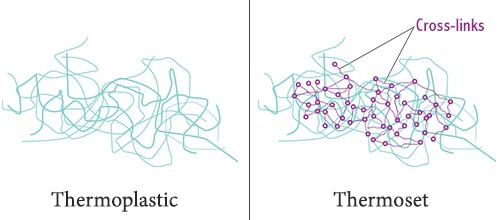
Polymers are divided into thermosets and thermoplastics.
One way of differentiating thermoplastics and thermosets involves how they react when heated:
- given sufficient heat, thermoplastics will soften and eventually melt back into a liquid state;
- thermosets remain solid, either charring or burning.
While this difference may seem simple, it has far reaching implications as far as the properties and behaviors of the two types of polymers.
Image Source
How Thermosets and Thermoplastics Cure
When thermosets cure, they undergo a process called cross-linking. Cross-linking forms a chemical bond between the polymer molecules that is irreversible. That is why a thermoset will char or burn rather than melt when exposed to high temperatures. This feature makes thermosets well adapted to applications involving elevated temperatures. Examples of thermosets include polyester resins, polyurethane, and phenolics.
When thermoplastics cure, on the other hand, there is no irreversible chemical bond taking place. Thermoplastics will harden without undergoing a chemical change. Instead, the molecules are held together by weaker Van Der Waals bonds. Because of this, thermoplastics will soften and melt when exposed to sufficiently high temperature. With sufficient heat they can be remolded easily and are usually recyclable. Examples of commonly used thermoplastics include ABS, PTFE, polycarbonate, nylon, and HDPE.
Major Characteristics of Thermosets
Thermosets typically have acceptable chemical and heat resistance, high stiffness, and dimensional stability. They are cheaper than thermoplastics, and more flexible when it comes to designs (e.g., ideal for both thick-walled and thin-walled geometries). Thermosets are also very resistant to creep.
Because of how the molecules chemically bond together, they are far more durable and dimensionally stable when exposed to high heat when compared to thermoplastics. They also tend to be more brittle and hard than thermoplastics.
Major Characteristics of Thermoplastics
Thermoplastics, on the other hand, are usually strong and tough and can be used in a wide range of applications, including those involving high stress. Their service temperatures are typically not as high as thermosets, however, and at very low temperatures they can become brittle.
Thermoplastics have very good chemical resistance and can be recycled. However, they are usually more expensive than thermosets.
Conclusion
The key difference between thermosets and thermoplastics lies in how they cure: thermosets undergo an irreversible chemical bonding called cross-linking while thermoplastics are loosely held together through Van Der Waals forces. This difference affects their strength, chemical resistance, operating temperatures, and flexibility when it comes to design.
{{cta(‘9c7fab19-3e70-4606-87dd-468123217802′,’justifycenter’)}}