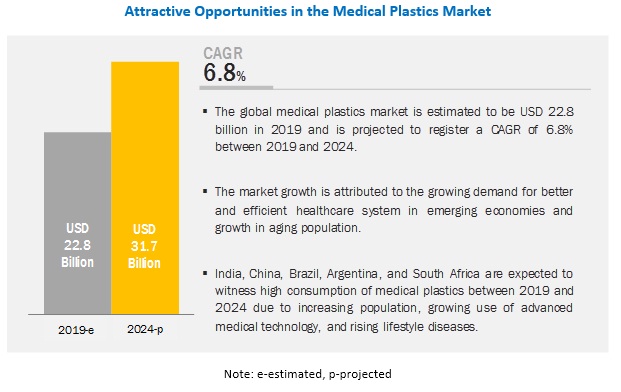
Spring-energized seals are a popular choice for applications that involve food, dairy, and medicinal applications, as well as medical devices. However, not just any polymer can be used for these seals because of the risk of contamination. In this blog post, we are going to review why FDA approved materials are so important, and then talk about the three most common engineering polymers that are used with spring-energized seals.
FDA Approved Materials
The FDA CFR 177 is contained in Title 21 of the Code of Federal Regulations and deals with indirect food additives in the form of polymers. The term indirect additive refers to something that inadvertently makes its way into food, pharmaceuticals, or inhaled substances (i.e., via an inhaler or oxygen machine).
Real-world applications where FDA approved materials are of concern include filling and mixing equipment for food and beverages as well as pharmaceutical products that can include pills, powders, caplets, tablets, liquids, oral suspensions, and inhalers. In the context of medical equipment, FDA approved materials are key to the design of devices that must be free of contamination. For analytical equipment it is vital that samples are not contaminated; machines and devices for treatment, such as blood dialysis machines or ventilators, must not have any contaminants that can harm the patient.
Spring Energized Seals with FDA Approved Materials
When spring-energized seals are combined with FDA approved materials, the result is a durable, reliable seal that is safe for use in food processing, pharmaceutical, and medical applications. Three of the most common materials used are UHMW PE, Virgin PTFE, and mineral-filled PTE
UMHW PE
UHMW PE (Ultra High Molecular Weight Polyethylene) is an FDA and USDA engineering polymer with an extremely low coefficient of friction, low moisture absorption, and good chemical compatibility. In addition, it can withstand rigorous sterilization requirements that may involve hot water, steam, or aggressive chemicals. One of its key characteristics is its self-lubrication, which eliminates the need for lubricants that could cause contamination issues.
Virgin PTFE
PTFE (Polytetrafluoroethylene) often goes by the trade name Teflon. Virgin PTFE, which has no fillers added, has the lowest coefficient of friction of any material in existence. It is also one of the most chemically inert engineering polymers and handles extreme temperatures (both cold and hot) and is both thermally and dimensionally stable. Like UHMW PE, it is also FDA and USDA approved as well as self-lubricating.
Mineral-Filled PTFE
Mineral-filled PTFE takes the outstanding properties of PTFE and enhances them for improved wear performance and strength. It, too, is FDA and USDA approved, self-lubricating, a wide range of operating temperature, chemical inertness, and compatibility with even the most aggressive cleaning and sterilization regimens.
Conclusion
Spring-energized seals provide the often mission-critical reliability that is demanded in industries involving food, medicine, and medical treatment. However, they must be matched with an FDA approved polymers such as UHMW PE, virgin PTFE, or mineral-filled PTFE.